
-

サイト内検索

分子内分泌学研究分野は、中外製薬株式会社の寄付講座であった脂溶性ビタミン研究分野からの組織改編により、新たに中外製薬株式会社、大正製薬株式会社、小野薬品株式会社、協和発酵キリン株式会社からの寄附講座として平成29年4月に藤井節郎記念医科学センター内に発足いたしました。本分野は、カルシウム代謝調節機能が知られる活性型ビタミンDを中心に、骨カルシウム代謝系に加え筋肉、皮膚、感染制御、膵β細胞分化・機能、脳高次機能、腫瘍増殖など多臓器に及ぶその多面的作用の解明を通じ、骨疾患、加齢に伴う筋萎縮、糖尿病、骨髄増殖性疾患や皮膚疾患など高齢社会における重要な疾患病態を解明し、新たな予防・治療法の開発に繋げる創薬基盤の構築を目的としています。さらにこれらの研究成果を、他の核内受容体を介する脂溶性リガンドの作用機序の解明や治療法開発への応用に繋げることを目指しています。

News
一覧を見るFGF23とリン感知機構に関する論文がPNASに掲載されました。
新規ゲノム編集ツールVIKING法に関する論文がScientific Reportsに掲載されました。
2018年1月12日、徳島大学および共同研究機関である立命館大学より報道発表しております。
VIKING法の詳細なプロトコールはこちら。
2018年も分子内分泌学研究分野をどうぞよろしくお願い申し上げます。
実り多き一年となりますよう、ラボメンバー一同精進してまいります。
福本がNature Reviews Disease Primersに腫瘍性骨軟化症の総説を発表いたしました。
高士がClinical Calciumに骨産生ホルモンに関する総説を執筆いたしました。
「Bone-derived hormones and their systemic regulation.」
沢津橋がClinical CalciumにビタミンDによる皮膚疾患治療に関する総説を執筆いたしました。
「Update on recent progress in vitamin D research. Vitamin D in the treatment of psoriasis.」
沢津橋がClinical Calciumに毛包幹細胞に関する総説を執筆いたしました。
「Hair follicle stem cells.」
特任研究員Maria K. Tsoumpra先生が2017年12月より、国立精神・神経医療研究センターに異動となりました。
今後の益々の活躍をラボメンバー一同祈念しております。
2017年度生命科学系学会合同年次大会(ConBio2017)(神戸ポートアイランド、2017年12月6日~9日)において、沢津橋が口演発表を、上甲がポスター発表いたしました。
沢津橋:「表皮特異的ビタミンD受容体ノックアウトマウスから理解する正常
上甲:「ビタミンD受容体は毛周期の成長期から退縮期への移行を
上甲が第3回Neo Vitamin D Workshop学術集会で優秀ポスター賞を受賞しました。
研究テーマ&内容
ビタミンD受容体の新規標的の探索
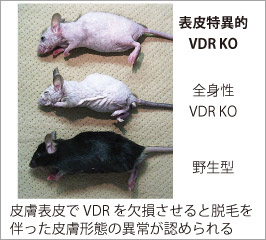 外因性異物から個体を保護するバリアである皮膚は、ビタミンDを生合成する唯一の臓器でもあり、産生されたビタミンDは内分泌系を介して体全体の健康状態と密接に関係しています。近年では骨代謝以外の分野においても高齢者の血中ビタミンDの低下が加齢に伴う様々な病変リスクに関与することが示されています。そこで我々は、ビタミンDの産生臓器かつ標的臓器である皮膚表皮におけるビタミンDの役割についてビタミンD受容体(Vitamin D receptor, VDR)を中心とした作用機序解明を進めています。 活性型ビタミンD(1,25-水酸化ビタミンD[1,25(OH)2D])はその受容体であるVDRに結合し、核内受容体型転写因子として遺伝子発現調節を介して、その生理作用を発揮します。我々の目標は、皮膚表皮特異的VDRノックアウト(KO)マウスの遺伝子発現解析を基に、表皮における活性型ビタミンDの新たな標的分子とその作用メカニズムを解明することです。この研究で得られる多くの知見は、現在活性型ビタミンDが治療薬として用いられる乾癬等の慢性炎症性皮膚疾患を標的とする創薬のみならず、加齢に伴う皮膚疾患治療への応用や、皮膚の抗老化を考慮したサプリメント・食物による栄養管理へと視野を拡げ、健康な皮膚を維持する為の応用へつながると期待しています。
外因性異物から個体を保護するバリアである皮膚は、ビタミンDを生合成する唯一の臓器でもあり、産生されたビタミンDは内分泌系を介して体全体の健康状態と密接に関係しています。近年では骨代謝以外の分野においても高齢者の血中ビタミンDの低下が加齢に伴う様々な病変リスクに関与することが示されています。そこで我々は、ビタミンDの産生臓器かつ標的臓器である皮膚表皮におけるビタミンDの役割についてビタミンD受容体(Vitamin D receptor, VDR)を中心とした作用機序解明を進めています。 活性型ビタミンD(1,25-水酸化ビタミンD[1,25(OH)2D])はその受容体であるVDRに結合し、核内受容体型転写因子として遺伝子発現調節を介して、その生理作用を発揮します。我々の目標は、皮膚表皮特異的VDRノックアウト(KO)マウスの遺伝子発現解析を基に、表皮における活性型ビタミンDの新たな標的分子とその作用メカニズムを解明することです。この研究で得られる多くの知見は、現在活性型ビタミンDが治療薬として用いられる乾癬等の慢性炎症性皮膚疾患を標的とする創薬のみならず、加齢に伴う皮膚疾患治療への応用や、皮膚の抗老化を考慮したサプリメント・食物による栄養管理へと視野を拡げ、健康な皮膚を維持する為の応用へつながると期待しています。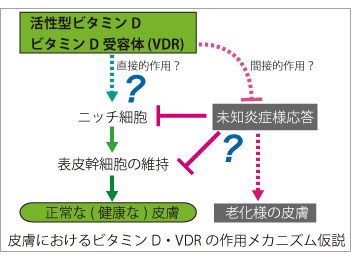 皮膚の発生研究を進めるFuchsらの報告によると、表皮幹細胞の維持に寄与するニッチ細胞でVDRは高発現する因子の一つであることが示されており(Tumbar et al. Science, 2004)、その詳細は全く明らかにされていませんが、VDRが正常に働かないと表皮幹細胞数の低下などの悪影響を与える可能性が考えられます。また我々の解析結果から、表皮特異的VDR KOマウスではいくつかの表皮幹細胞胞マーカー遺伝子の発現量低下が認められ、VDRがin vivoにおける表皮幹細胞の維持因子として機能する可能性を示唆しています。さらに、このマウスでは胎児期に生えた体毛が生後に抜け落ちても毛周期が停止し続けるため、新たな毛が全く生えないことが判明しており、現在このような表現型を呈する原因分子を探っています。最近では内在性の炎症様応答が惹起されているものと予想される結果が得られており、さらなる検討を進めています。
皮膚の発生研究を進めるFuchsらの報告によると、表皮幹細胞の維持に寄与するニッチ細胞でVDRは高発現する因子の一つであることが示されており(Tumbar et al. Science, 2004)、その詳細は全く明らかにされていませんが、VDRが正常に働かないと表皮幹細胞数の低下などの悪影響を与える可能性が考えられます。また我々の解析結果から、表皮特異的VDR KOマウスではいくつかの表皮幹細胞胞マーカー遺伝子の発現量低下が認められ、VDRがin vivoにおける表皮幹細胞の維持因子として機能する可能性を示唆しています。さらに、このマウスでは胎児期に生えた体毛が生後に抜け落ちても毛周期が停止し続けるため、新たな毛が全く生えないことが判明しており、現在このような表現型を呈する原因分子を探っています。最近では内在性の炎症様応答が惹起されているものと予想される結果が得られており、さらなる検討を進めています。また我々は皮膚に加え、筋組織における活性型ビタミンDの作用メカニズムについても解析を進めています。近年、ビタミンD不足は筋量および筋機能低下を特徴とするサルコペニア(Sarcopenia)発症の一因として注目されており、筋肉におけるVDRの作用機序を解明することで、筋機能低下を伴う運動機能障害の新たな治療標的の創出につながるものと期待します。
グルココルチコイド受容体の機能調節機構の解明
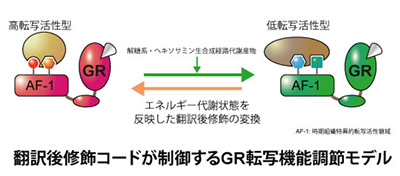 近年増加し続ける生活習慣病に起因する代謝異常と持続的なストレス応答が慢性的な炎症を惹起し様々な疾患に繋がることから、この栄養代謝系の変化が炎症状態の悪循環を促すメカニズムを明らかにすることは火急の課題といえます。なかでもストレスに対する防御応答として分泌されるグルココルチコイドは、糖新生やタンパク異化促進などの栄養代謝調節に働くホルモンとしての多面的な生理作用と、臨床での強力な抗炎症薬としての薬理作用の両側面において重要な働きを担っています。我々は、「栄養代謝制御」と「炎症制御」の二つの役割を担う核内受容体グルココルチコイドレセプター(GR)を中心に、栄養状態がもたらすエネルギー代謝系からの分子情報が翻訳後修飾の形で集積し、“転写環境”を形成する新たなクロストークメカニズムの解明を目指しています。特に解糖系やヘキソサミン生合成経路などから産生されるアセチルCoA、UDP-GlcNAc等の代謝産物がもたらすGRの多重の翻訳後修飾の変動に着目し、栄養状態が抗炎症作用をはじめとするグルココルチコイド感受性を制御する可能性について検討しています。我々のこれまでの解析から、複数のエピゲノム制御因子がこの多重の翻訳後修飾に関与することが示唆されています。このようなエピゲノム制御因子による翻訳後修飾の解析では、生化学的手法による標的タンパク質の精製と質量分析装置を利用したプロテオミクス解析を用いることで翻訳後修飾の種類・部位の同定ができ、そのような分子情報を解明することにより、将来的にはグルココルチコイド作用をコントロールする方法の開発につながると期待しています。
近年増加し続ける生活習慣病に起因する代謝異常と持続的なストレス応答が慢性的な炎症を惹起し様々な疾患に繋がることから、この栄養代謝系の変化が炎症状態の悪循環を促すメカニズムを明らかにすることは火急の課題といえます。なかでもストレスに対する防御応答として分泌されるグルココルチコイドは、糖新生やタンパク異化促進などの栄養代謝調節に働くホルモンとしての多面的な生理作用と、臨床での強力な抗炎症薬としての薬理作用の両側面において重要な働きを担っています。我々は、「栄養代謝制御」と「炎症制御」の二つの役割を担う核内受容体グルココルチコイドレセプター(GR)を中心に、栄養状態がもたらすエネルギー代謝系からの分子情報が翻訳後修飾の形で集積し、“転写環境”を形成する新たなクロストークメカニズムの解明を目指しています。特に解糖系やヘキソサミン生合成経路などから産生されるアセチルCoA、UDP-GlcNAc等の代謝産物がもたらすGRの多重の翻訳後修飾の変動に着目し、栄養状態が抗炎症作用をはじめとするグルココルチコイド感受性を制御する可能性について検討しています。我々のこれまでの解析から、複数のエピゲノム制御因子がこの多重の翻訳後修飾に関与することが示唆されています。このようなエピゲノム制御因子による翻訳後修飾の解析では、生化学的手法による標的タンパク質の精製と質量分析装置を利用したプロテオミクス解析を用いることで翻訳後修飾の種類・部位の同定ができ、そのような分子情報を解明することにより、将来的にはグルココルチコイド作用をコントロールする方法の開発につながると期待しています。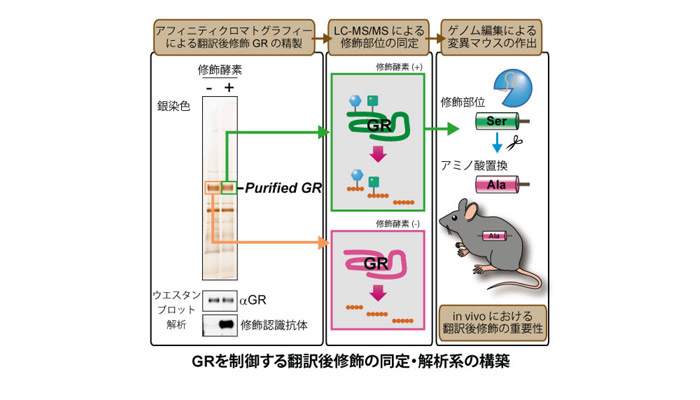
ミネラル代謝調節機構の解明
血中カルシウム(Ca)やリン濃度の異常は、筋肉痙攣や意識障害、異所性石灰化、骨石灰化障害を特徴とするくる病や骨軟化症など、種々の病変の原因となります。これらの病態を惹起しないように、生体には血中Caやリン濃度を一定の範囲に維持する機構が備わっています。このうち血中Ca濃度の維持には、Ca調節ホルモンである副甲状腺ホルモン(parathyroid hormone: PTH)と1,25-水酸化ビタミンD[1,25(OH)2D]の作用が必須であることが知られていました。従来我々は、この血中Caやリン濃度調節機構に関する検討を行い、以下のことなどを発表してきました。| 1. | PTH分泌調節に重要な役割を果たすCa感知受容体遺伝子が二つのプロモーターを有し、副甲状腺腺腫ではこのうち一つのプロモーターからのCaSR遺伝子発現が低下している。 |
|---|---|
| 2. | CaSR遺伝子活性型変異が、ナトリウム喪失性腎症の一つであるBartter症候群5型の原因となる。 |
| 3. | 低リン血症を特徴とする腫瘍随伴症候群である腫瘍性骨軟化症の惹起因子として、線維芽細胞増殖因子23(fibroblast growth factor 23: FGF23)をクローニング。 |
| 4. | FGF23測定系の確立。 |
| 5. | FGF23の作用を明らかにし、FGF23がリン調節ホルモンとして機能することを証明。 |
| 6. | 血中FGF23濃度の測定が、低リン血症性疾患の病因鑑別に有用である。 |
| 7. | 過剰なFGF23作用は、いくつかの低リン血症性疾患の原因となる。 |
| 8. | Klotho-FGF受容体が、FGF23に対する受容体として機能する(図)。 |
| 9. | FGF23作用障害が、高リン血症性腫瘍状石灰沈着症の原因となる。 |
| 10. | 抗FGF23抗体によるFGF23作用阻害が、過剰なFGF23作用による低リン血症性疾患に対する新たな治療法と成る可能性がある。 |
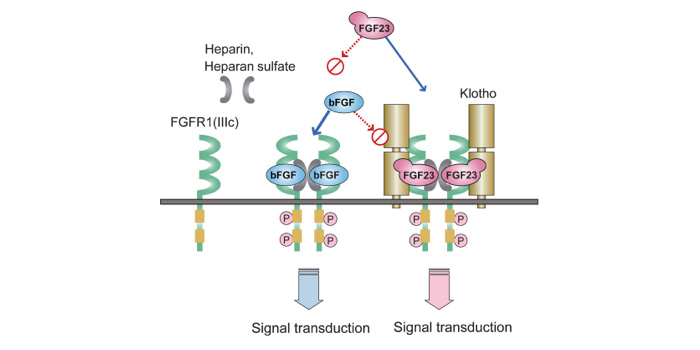 これらの背景のもと、現在FGF23産生調節機構やFGF23蛋白の翻訳後修飾、各種組織における1,25(OH)2D作用についての検討を進めている。
これらの背景のもと、現在FGF23産生調節機構やFGF23蛋白の翻訳後修飾、各種組織における1,25(OH)2D作用についての検討を進めている。メンバー紹介 ※スパムメール対策の為、@マークを(@)とさせていただいております。
- 特任教授
福本 誠二
(ふくもと せいじ) - TEL:088-634-6440
Email:fukumoto.seiji.1(@)tokushima-u.ac.jp
- 特任講師
沢津橋 俊
(さわつばし しゅん) - TEL:088-634-6417
Email:shun-sawa2(@)tokushima-u.ac.jp
- 技術補佐員
坂井 利佳
(さかい りか) - TEL:
Email:rsakai00(@)tokushima-u.ac.jp
- 技術補佐員
三浦 直子
(みうら なおこ) - TEL:088-634-6404
Email:miura.naoko(@)tokushima-u.ac.jp
- 大学院生
上甲 裕大
(じょうこう ゆうだい) - TEL:
Email:c501304057(@)tokushima-u.ac.jp
- 大学院生
貝沼梨沙
(かいぬま りさ) - TEL:
Email:c201956009@tokushima-u.ac.jp

- センター顧問
松本 俊夫
(まつもと としお) - TEL:088-634-6400
Email:toshio.matsumoto(@)
tokushima-u.ac.jp

- 秘書
武市 弘実
(たけいち ひろみ) - TEL:088-634-6426
Email:h.takeichi(@)
tokushima-u.ac.jp
主な発表論文(2014~)
- Takashi Y, Kosako H, Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK, Nangaku M, Abe M, Matsuhisa M, Kato S, Matsumoto T, Fukumoto S: Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci U S A. 116:11418-11427, 2019.
- Takashi Y, Fukumoto S: FGF23 beyond Phosphotropic Hormone. Trends Endocrinol Metab. 29:755-767, 2018
- Kobayashi H, Akiyama T, Okuma T, Shinoda Y, Oka H, Ito N, Fukumoto S, Tanaka S, Kawano H: Three-dimensional fluoroscopic navigation-assisted surgery for tumors in patients with tumor-induced osteomalacia in the bones. Comput Assist Surg 22: 14-19, 2017.
- Takashi Y, Kinoshita Y, Hori M, Ito N, Taguchi M, Fukumoto S: Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res. 42:132-137, 2017.
-
Kinoshita Y, Arai M, Ito N, Takashi Y, Makita N, Nangaku M, Shinoda Y, Fukumoto S: High serum ALP level is associated with increased risk of denosumab-related hypocalcemia in patients with bone metastases from solid tumors. Endocr J 63: 479-484, 2016.
-
Tajima S, Takashi Y, Ito N, Fukumoto S, Fukayama M: ERG and FLI1 are useful immunohistochemical markers in phosphaturic mesenchymal tumors. Med Mol Morphol 49: 203-209, 2016.
-
Fukumoto S: FGF23-FGF receptor-Klotho pathway as a new drug target for disorders of bone and mineral metabolism. Calcif Tissue Int 98: 334-340, 2016.
-
Endo I, Fukumoto S, Ozono K, Namba N, Inoue D, Okazaki R, Yamauchi M, Sugimoto T, Minagawa M, Michigami T, Nagai M, Matsumoto T: Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J 62: 811-816, 2015.
-
Dong B, Endo I, Ohnishi Y, Kondo T, Hasegawa T, Amizuka N, Kiyonari H, Shioi G, Abe M, Fukumoto S, Matsumoto T: Calcilytic ameliorates abnormalities of mutant calcium-sensing receptor (CaSR) knock-in mice mimicking autosomal dominant hypocalcemia (ADH). J Bone Miner Res 30: 1980-1993, 2015.
-
Kinoshita Y, Hori M, Taguchi M, Watanabe S and Fukumoto S: Functional Activities of Mutant Calcium-Sensing Receptors Determine Clinical Presentations in Patients With Autosomal Dominant Hypocalcemia. J Clin Endocrinol Metab 99: E363-E368, 2014.
-
Kinoshita Y, Hori M, Taguchi M and Fukumoto S: Functional analysis of mutant FAM20C in Raine syndrome with FGF23-related hypophosphatemia. Bone 67: 145-151, 2014.
-
Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T and Shimosawa T: Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int 85: 1103-1111, 2014.
主な発表論文(~2013)
-
Vandyke K, Fitter S, Drew J, Fukumoto S, Schultz CG, Sims NA, Yeung DT, Hughes TP and Zannettino AC: Prospective histomorphometric and DXA evaluation of bone remodeling in imatinib-treated CML patients: evidence for site-specific skeletal effects. J Clin Endocrinol Metab 98: 67-76, 2013.
-
Kinoshita Y, Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S and Fujita T: Mutational analysis of patients with FGF23-related hypophosphatemic rickets. Eur J Endocrinol 167: 165-172, 2012.
-
Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S and Fujita T: A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone 49: 913-916, 2011.
-
Hori M, Shimizu Y and Fukumoto S: Minireview: Fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 152: 4-10, 2011.
-
Aono Y, Hasegawa H, Yamazaki Y, Shimada T, Fujita T, Yamashita T and Fukumoto S: Anti-FGF23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res 26: 803-810, 2011.
-
Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya KI and Takeda S: A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A 106: 20794-20799, 2010.
-
Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S and Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975-980, 2010.
-
Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, Fukumoto S, Sugimoto T and Fujita T: Hypophosphatemia induced by intravenous administration of saccharated ferric oxide -Another form of FGF23-related hypophosphatemia. Bone 45: 814-816, 2009.
-
Fukumoto S and Martin TJ: Bone as an endocrine organ. Trends Endocrinol Metab 20: 230-236, 2009.
-
Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S and Shimada T: Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res 24: 1879-1888, 2009.
-
Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S and Shimada T: Anti-FGF23 neutralizing antibodies demonstrate the physiological role and structural features of FGF23. J Bone Miner Res 23: 1509-1518, 2008.
-
Sato S, Kimura A, Ozdemir J, Asou Y, Miyazaki M, Jinno T, Ae K, Liu X, Osaki M, Takeuchi Y, Fukumoto S, Kawaguchi H, Haro H, Shinomiya K, Karsenty G and Takeda S: The distinct role of the runx proteins in chondrocyte differentiation and intervertebral disc degeneration. Arthritis Rheum 58: 2764-2775, 2008.
24) Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T and Matsumoto T: Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients – Proposal of diagnostic criteria using FGF23 measurement-. Bone 42: 1235-1239, 2008.
-
Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya KI and Takeda S: Central control of bone remodelling by neuromedin U. Nat Med 13: 1234-1240, 2007.
-
Fukumoto S and Yamashita T: FGF23 is a hormone regulating phosphate metabolism -Unique biological characteristics of FGF23-. Bone 40: 1190-1195, 2007.
-
Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T and Fukumoto S: Hyperostosis – hyperphosphatemia syndrome: A congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 27: 235-242, 2007.
-
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S and Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770-774, 2006.
-
Yamashita H, Yamazaki Y, Hasegawa H, Yamashita T, Fukumoto S, Shigematsu T, Kazama JJ, Fukagawa M and Noguchi S: Fibroblast growth factor (FGF)-23 in patients with Graves’ disease before and after antithyroid therapy: Its important role in serum phosphate regulation. J Clin Endocrinol Metab 90: 4211-4215, 2005.
-
Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S and Yamashita T: Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289: F1088-F1095, 2005.
-
Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T and Fukagawa M: Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int 67: 1171-1178, 2005.
-
Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S and Fukagawa M: Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int 67: 1120-1125, 2005.
-
Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T and Fujita T: A novel mutation in fibroblast growth factor (FGF)23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab 90: 5523-5527, 2005.
—————————————————————————————————————————————– -
Ito S, Fujiyama-Nakamura S, Kimura S, Lim J, Kamoshida Y, Shiozaki-Sato Y, Sawatsubashi S, Suzuki E, Tanabe M, Ueda T, Murata T, Kato H, Ohtake F, Fujiki R, Miki T, Kouzmenko A, Takeyama K and Kato S: Epigenetic silencing of core histone genes by HERS in Drosophila. Mol Cell 45: 494-504, 2012.
-
Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E, Tanabe M, Zhao Y, Kimura S, Fujiyama S, Ueda T, Umetsu D, Ito T, Takeyama K and Kato S: A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev 24: 159-170, 2010.
-
Zhao Y, Takeyama K, Sawatsubashi S, Ito S, Suzuki E, Yamagata K, Tanabe M, Kimura S, Fujiyama S, Ueda T, Murata T, Matsukawa H, Shirode Y, Kouzmenko AP, Li F, Tabata T and Kato S: Corepressive action of CBP on androgen receptor transactivation in pericentric heterochromatin in a Drosophila experimental model system. Mol Cell Biol 29: 1017-1034, 2009.
-
Suzuki E, Zhao Y, Ito S, Sawatsubashi S, Murata T, Furutani T, Shirode Y, Yamagata K, Tanabe M, Kimura S, Ueda T, Fujiyama S, Lim J, Matsukawa H, Kouzmenko AP, Aigaki T, Tabata T, Takeyama K and Kato S: Aberrant E2F activation by polyglutamine expansion of androgen receptor in SBMA neurotoxicity. Proc Natl Acad Sci U S A 106: 3818-3822, 2009.
-
Fujiyama-Nakamura S, Ito S, Sawatsubashi S, Yamauchi Y, Suzuki E, Tanabe M, Kimura S, Murata T, Isobe T, Takeyama K and Kato S: BTB protein, dKLHL18/CG3571, serves as an adaptor subunit for a dCul3 ubiquitin ligase complex. Genes Cells 14: 965-973, 2009.
-
Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG, Krasnov A, Georgieva SG, Schule R, Takeyama K, Kato S, Tora L and Devys D: A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell 29: 92-101, 2008.
-
Murata T, Suzuki E, Ito S, Sawatsubashi S, Zhao Y, Yamagata K, Tanabe M, Fujiyama S, Kimura S, Ueda T, Matsukawa H, Kouzmenko A, Furutani T, Kuranaga E, Miura M, Takeyama K and Kato S: RNA-binding protein hoip accelerates polyQ-induced neurodegeneration in Drosophila. Biosci Biotechnol Biochem 72: 2255-2261, 2008.
-
Kimura S, Sawatsubashi S, Ito S, Kouzmenko A, Suzuki E, Zhao Y, Yamagata K, Tanabe M, Ueda T, Fujiyama S, Murata T, Matsukawa H, Takeyama K, Yaegashi N and Kato S: Drosophila arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. Biochem Biophys Res Commun 371: 889-893, 2008.
-
Sawatsubashi S, Maki A, Ito S, Shirode Y, Suzuki E, Zhao Y, Yamagata K, Kouzmenko A, Takeyama K and Kato S: Ecdysone receptor-dependent gene regulation mediates histone poly(ADP-ribosyl)ation. Biochem Biophys Res Commun 320: 268-272, 2004.
-
Maki A, Sawatsubashi S, Ito S, Shirode Y, Suzuki E, Zhao Y, Yamagata K, Kouzmenko A, Takeyama K and Kato S: Juvenile hormones antagonize ecdysone actions through co-repressor recruitment to EcR/USP heterodimers. Biochem Biophys Res Commun 320: 262-267, 2004.
-
Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T and Kato S: Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem 279: 40255-40258, 2004.
—————————————————————————————————————————————–
Recknor CP, Recker RR, Benson CT, Robins DA, Chiang AY, Alam J, Hu L, Matsumoto T, Sowa H, Sloan JH, Konrad RJ, Mitlak BH and Sipos AA: The Effect of Discontinuing Treatment with Blosozumab: Follow-up Results of a Phase 2 Randomized Clinical Trial in Postmenopausal Women with Low Bone Mineral Density. J Bone Miner Res 2015. -
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH and Myers SL: A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30: 216-224, 2015.
-
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M and Fukunaga M: Fracture Risk Reduction with Denosumab in Japanese Postmenopausal Women and Men with Osteoporosis: Denosumab fracture Intervention RandomizEd placebo Controlled Trial (DIRECT). J Clin Endocrinol Metab 99: 2599-2607, 2014.
-
Matsumoto T., Takano T, Saito H and Takahashi F: Vitamin D analogs and bone: preclinical and clinical studies with eldecalctol. BoneKEy Reports 3: Article number 513, 2014.
-
Yoshida S, Aihara K, Ikeda Y, Sumitomo-Ueda Y, Uemoto R, Ishikawa K, Ise T, Yagi S, Iwase T, Mouri Y, Sakari M, Matsumoto T, Takeyama K, Akaike M, Matsumoto M, Sata M, Walsh K, Kato S and Matsumoto T: Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation 128: 60-71, 2013.
-
Kuriwaka-Kido R, Kido S, Miyatani Y, Ito Y, Kondo T, Omatsu T, Dong B, Endo I, Miyamoto K and Matsumoto T: Parathyroid hormone (1-34) counteracts the suppression of interleukin-11 expression by glucocorticoid in murine osteoblasts: a possible mechanism for stimulating osteoblast differentiation against glucocorticoid excess. Endocrinology 154: 1156-1167, 2013.
-
Matsumoto T, Kuriwaka-Kido R, Kondo T, Endo I and Kido S: Regulation of osteoblast differentiation by interleukin-11 via AP-1 and Smad signaling [Review]. Endocr J 59: 91-101, 2012.
-
Ikegame A, Ozaki S, Tsuji D, Harada T, Fujii S, Nakamura S, Miki H, Nakano A, Kagawa K, Takeuchi K, Abe M, Watanabe K, Hiasa M, Kimura N, Kikuchi Y, Sakamoto A, Habu K, Endo M, Itoh K, Yamada-Okabe H and Matsumoto T: Small molecule antibody targeting HLA class I inhibits myeloma cancer stem cells by repressing pluripotency-associated transcription factors. Leukemia 26: 2124-2134, 2012.
-
Ikeda Y, Aihara KI, Yoshida S, Iwase T, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Sata M, Akaike M, Kato S, Matsumoto T and Tamaki T: Heparin cofactor II, a serine protease inhibitor, promotes angiogenesis via activation of the AMPK-eNOS signaling pathway. J Biol Chem 2012.
-
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M and Nakamura T: A new active vitamin D(3) analog, eldecalcitol, prevents the risk of osteoporotic fractures – A randomized, active comparator, double-blind study. BONE 49: 605-612, 2011.
-
Matsumoto T and Abe M: TGF-β-related mechanisms of bone destruction in multiple myeloma. Bone 48: 129-134, 2011.
-
Asano J, Nakano A, Oda A, Amou H, Hiasa M, Takeuchi K, Miki H, Nakamura S, Harada T, Fujii S, Kagawa K, Endo I, Yata K, Sakai A, Ozaki S, Matsumoto T and Abe M: The serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in myeloma cells. Leukemia 25: 1182-1188, 2011.
-
Mihara M, Aihara K, Ikeda Y, Yoshida S, Kinouchi M, Kurahashi K, Fujinaka Y, Akaike M and Matsumoto T: Inhibition of thrombin action ameliorates insulin resistance in type 2 diabetic db/db mice. Endocrinology 151: 513-519, 2010.
-
Kido S, Kuriwaka-Kido R, Imamura T, Ito Y, Inoue D and Matsumoto T: Mechanical stress induces Interleukin-11 expression to stimulate osteoblast differentiation. BONE 45: 1125-1132, 2009.
-
Ikeda Y, Aihara K, Yoshida S, Sato T, Yagi S, Iwase T, Sumitomo Y, Ise T, Ishikawa K, Azuma H, Akaike M, Kato S and Matsumoto T: Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology 150: 2857-2864, 2009.
-
Hiasa M, Abe M, Nakano A, Oda A, Amou H, Kido S, Takeuchi K, Kagawa K, Yata K, Hashimoto T, Ozaki S, Asaoka K, Tanaka E, Moriyama K and Matsumoto T: GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE). Blood 114: 4517-4526, 2009.
-
Yagi S, Aihara K, Ikeda Y, Sumitomo Y, Iwase T, Ishikawa K, Azuma H, Akaike M and Matsumoto T: Pitavastatin, an HMG-CoA Reductase Inhibitor, Exerts eNOS-independent Protective Actions against Angiotensin II-Induced Cardiovascular Remodeling and Renal Insufficiency. Circ Res 102: 68-76, 2008.
-
Sekimoto E, Ozaki S, Ohshima T, Shibata H, Hashimoto T, Abe M, Kimura N, Hattori K, Kawai S, Kinoshita Y, Yamada-Okabe H, Tsuchiya M and Matsumoto T: A single chain Fv diabody against HLA-A molecules specifically induces myeloma cell death in the bone marrow environment. Cancer Res 67: 1184-1192, 2007.
-
Obata T, Yokota I, Yokoyama K, Okamoto E, Kanezaki Y, Tanaka Y, Maegawa H, Teshigawara K, Hirota F, Yuasa T, Kishi K, Hattori A, Hashida S, Masuda K, Matsumoto M, Matsumoto T, Kashiwagi A and Ebina Y: Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes 56: 2028-2035, 2007.
-
Aihara K, Azuma H, Akaike M, Ikeda Y, Sata M, Takamori N, Yagi S, Iwase T, Sumitomo Y, Kawano H, Yamada T, Fukuda T, Matsumoto T, Sekine K, Sato T, Nakamichi Y, Yamamoto Y, Yoshimura K, Watanabe T, Nakamura T, Oomizu A, Tsukada M, Hayashi H, Sudo T, Kato S and Matsumoto T: Strain-dependent embryonic lethality and exaggerated vascular remodeling in heparin cofactor II-deficient mice. J Clin Invest 117: 1486-1489, 2007.
-
Matsumoto T, Shiraki M, Hagino H, Iinuma H and Nakamura T: Daily nasal spray of hPTH(1-34) for 3 months increases bone mass in osteoporotic subjects: a pilot study. Osteoporos Int 17: 1532-1538, 2006.
-
Matsumoto T and Abe M: Myeloma-Bone Interactioon: A Vicious Cycle. BoneKEy-Osteovisioon 3: 8-14, 2006.
-
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D and Matsumoto T: Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106: 3160-3165, 2005.
-
Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M and Nakamura T: A New Active Vitamin D, ED-71, Increases Bone Mass in Osteoporotic Patients under Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Clin Endocrinol Metab 90: 5031-5036, 2005.
-
Jalili A, Ozaki S, Hara T, Shibata H, Hashimoto T, Abe M, Nishioka Y and Matsumoto T: Induction of HM1.24 peptide-specific cytotoxic T lymphocytes by using peripheral-blood stem-cell harvests in patients with multiple myeloma. Blood 106: 3538-3545, 2005.
フォトギャラリー










